Promotional & Advertising Opportunities
PROMOTIONAL OPPORTUNITIES

- Opportunity to organize an Official Non-CME Industry Session in a Plenary Hall, up to 60 minutes and inclusive of Lunch (Program subject to the approval by the Committee).
- Includes hall rental, standard audio/visual equipment, and display table.
- Permission to use the phrase “Official Symposium of the WOM 22 Congress”.
- Sponsored Symposia Programs will be included in a designated industry section of the Final Programme (subject to receipt by publishing deadline).
- Time Slots: allocated on a first come, first served basis – see time slots here.
- Industry sessions will be clearly indicated in the meeting timetable/Programme as: “Industry Session” not included in the main event CME/CPD credit offering”.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.
NOTE: The supporting company in addition to the support fee must cover all speakers’ expenses including:
- Registration fee
- Accommodation
- Travel expenses
This also applies in the case where the speakers have already been invited by the Congress. In this case, the company will support the amount of nights as per congress policy.

- Opportunity to present/talk in an Official Non-CME Industry Session in the Main Program or in Parallel to the Main Program for 20/30 minutes
- Permission to use the phrase “Official Symposium of the WOM 2022 Congress”.
- Sponsored Symposia Programs will be included in a designated industry section of the Final Programme (subject to receipt by publishing deadline).
- Time Slots: allocated on a first come, first served basis
- Industry sessions will be clearly indicated in the meeting timetable/Programme as: “Industry Session” not included in the main event CME/CPD credit offering”.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.
NOTE: The supporting company in addition to the support fee must cover all speakers’ expenses including:
- Registration fee
- Accommodation
- Travel expenses
This also applies in the case where the speakers have already been invited by the Congress. In this case, the company will support the amount of nights as per congress policy.
- Pre-congress company workshop session up to 60 minutes, gives you the opportunity to demonstrate your new technologies or devices in front of industry stakeholders & decision makers
- Program subject to the approval of the Conference Scientific Committee.
- Includes: hall rental, standard audio/visual equipment, display table.
- Permission to use the phrase: “Official workshop of the 3rd edition of WOM22”.
- Workshop Programs will be included in a designated industry section of the Final Programme (subject to receipt by publishing deadline).
- Supporters will be acknowledged in a designated section of the Program.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.
This also applies in the case where the speakers have already been invited by the Congress. In this case, the company will support the amount of nights as per congress policy.

Meet with attendees and key decision makers to share your new research outcomes, discuss your clinical protocols, and conduct product demonstrations of your new products and services. Product Theatre sessions are 10 minutes in length and will be held in a designated area(s) in the exhibit hall, which is set up in theater style for 50 attendees. No other sessions of the scientific programme will run in parallel but may run concurrent with other corporate sponsors.
Product Theaters provide a high value, live educational opportunity for hosts to reach engaged healthcare professionals. These sessions deliver a platform to gather and discuss issues on patient education, specific products and therapeutic areas
Located in the Exhibit hall, Product Theatre provides an opportunity to:
- Highlight and demonstrate new and existing products.
- Provide up-to-date research findings.
- Give product details in-depth.
- Demonstrate products.
- Distribute branded materials.

Opportunity to place company logo on the lanyards. The Organizing Committee will select the type and design of the lanyards. The support entitlements are as follows:
- Supporter’s logo to be printed on the lanyards.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.

Supporter will provide funding for the Notepads & Pens for the participants.
- Notepads & Pens will bear the WOM22 logo and the Supporter’s company logo and will be distributed in the participants’ Conference bags.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event

Supporter will provide funding of the Conference bags.
- The bag will bear the Supporter’s logo and the Conference logo
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.
* The bag must be approved by the organizing committee in advance.

Coffee/Refreshments will be served during breaks in the exhibition area. Hospitality provided will be in compliance with all relevant industry codes.
- Opportunity to provide items bearing company logo for use during the supported break.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.
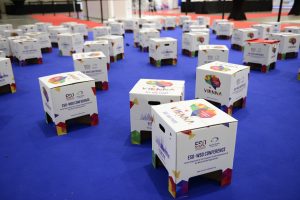
The branded Seating Cubes or Festival Chairs are stylish and informal. This multipourpose cardboard stool can be customized to match whatever theme you have chosen for your event. The design is subject to approval of the Secretariat and must follow all compliance regulations.
- Opportunity to customize the seating cubes.
- 50 or 100 branded seats will be produced, price is according to the amount.
- Location of seating cubes onsite to be coordinated with Secretariat.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the even

Different options for branding in the venue will be offered upon request.

Supporter will have the opportunity to promote itself through a networking reception on the first evening to which all registered attendees are invited. Hospitality and any activities provided will be in compliance with all relevant industry codes.
- Supporter’s logo on sign at the entrance to the Welcome Reception.
- Opportunity to provide items bearing company logo for use at the event.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.
ADVERTISING OPPORTUNITIES

- The Final Programme will contain the timetable, information about the scientific Program and other useful information. It will be distributed to all registered participants in the Conference bags.
- The advertisement will be printed in the designated industry section of the programme, according to compliance regulations.
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.

Promotional material (up to 4-page insert, A5 flyer) will be included in the Meeting bags.
- Material should be provided by the Supporter and approved by the Secretariat.
- Supporters’ product information will be available for all Conference participants.
- The distribution arrangement will be advised.

Gain additional exposure for your Symposium, company or exhibition booth by sending out a Mail Blast to the pre-registered delegates who have agreed to receive promotional material, at a date and time coordinated with the Congress Organizer.
- Exclusive: Mail blast will be exclusive for the supporting company. The designed mail blast (html format with Kenes design requirements) and the preferred “Subject” to be provided by the Supporter and subject to receipt by 6 weeks prior to the Congress. ”From” field will be Congress Acronym + Year .
- Joint: Mail blast will be shared with other supporting companies. Supporting company should provide the content for the mail blast following Kenes design requirements. Design of mail blast will be done by Kenes/Organizer.
*In the case where the supporter cannot provide a compliant HTML file, the may provide one pdf/ image, that will be embedded into the congress designed mailshot for an additional charge of $ 250. Content received after the deadline may be processed for an additional fee of $500.
Industry Support Disclosure – will be added to all mailshots
This event is supported, in part, by funding from industry. All support is managed in strict accordance with CME/CPD accreditation criteria and standards for commercial support. Industry Sponsored Symposia are organized by industry and not included in the main event CME/CPD credit offering.
An example to a joint mailshot:

Gain additional exposure for your repeat industry session by sending out a post-Congress Exclusive Mail Blast to registered delegates who have agreed to receive promotional material, at a date and time coordinated with the Congress Organizer.
- Mail blast will be exclusive for the supporting company. The designed mail blast (html format with Kenes design requirements) and the preferred “Subject” to be provided by the Supporter.
*In the case where the supporter cannot provide a compliant HTML file, the may provide one pdf/ image, that will be embedded into the congress designed mailshot for an additional charge of $ 250. Content received after the deadline may be processed for an additional fee of $500.
Industry Support Disclosure – will be added to all mailshots
This event is supported, in part, by funding from industry. All support is managed in strict accordance with CME/CPD accreditation criteria and standards for commercial support. Industry Sponsored Symposia are organized by industry and not included in the main event CME/CPD credit offering.


- Support will be acknowledged on on inside back cover as: ˝Supported by… ˝ and a company logo only
- Support will be acknowledged in the Industry Support and Exhibition section of the program guide, on the event website and application, and with signage during the event.

- Gain additional exposure for your Company participating in WOM 2022 by adding a dedicated social media post in your package.
- Our Marketing Team will take care to post a message, prepared by you, in the social media channels of the congress.
Please note that it is the Exhibitors’/ Supporters’ responsibility to comply with the local authority’s regulations, EFPIA (European Federation of Pharmaceuticals Industries & Associations) www.efpia.org, Medtech Europe (represents Medical Technology industry) http://www.medtecheurope.org/ and IFPMA (International Federation of Pharmaceutical Manufacturers & Associations) www.ifpma.org Code of Practice on the promotion of medicines. Failure to comply with these regulations may not be used as a ground to declare the contract void. Failure to comply with the rules and regulations will not expose the Organizer to any suits, demands by the Exhibitor/Supporter or any other third party.
REGULATIONS
Please note that it is the Exhibitors’ and/or Supporters’ responsibility to comply with the local authority’s regulations, including, without limitation, IFPMA, the International Federation of Pharmaceutical Manufacturers & Associations Code of Practice on the promotion of medicines (www.ifpma.org), as well as FDA restrictions on the promotion of investigational and preapproved drugs and devices and the FDA prohibition on promoting approved drugs and devices for unapproved uses. Any product not FDA-approved for a particular use or not commercially available in the U.S. may be exhibited only if accompanied by easily visible signs indicating the status of the product.
Failure to comply with these regulations may not be used as a ground to declare the contract void. Failure to comply with the rules and regulations will not expose the Organizer to any suits, demands by the Exhibitor/Supporter or any other third party.
SPECIAL REQUESTS
Tailored packages can be arranged to suit your objectives. Please do not hesitate to contact me to discuss your needs:
Marieta Tseneva – Industry Liaison & Sales Associate
Email: mtseneva@kenes.com
ACKNOWLEDGEMENTS
Support will be recognized in the Industry Support and Exhibition section of the programme, on the event website, mobile application and with signage during the event.
*All pictures are illustrations only.
Contact us now
for pricing, bookings and customized packages.
